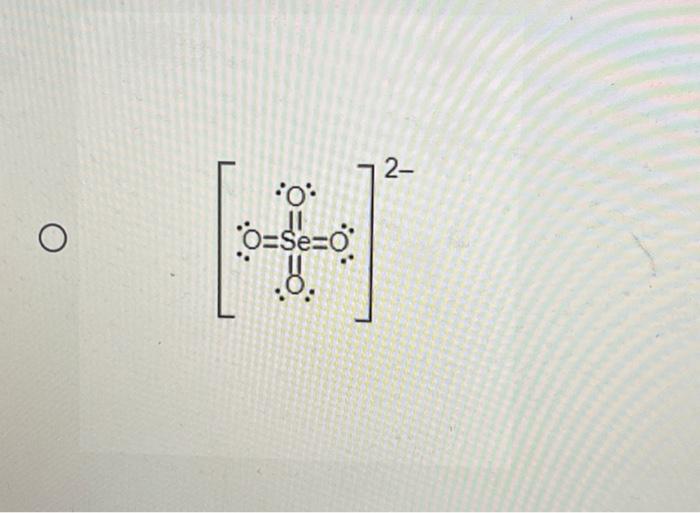
Then, learn how to draw the lewis structure. · to draw the lewis structure for the trisulfide anion (s₃²⁻), count a total of 20 valence electrons from three sulfur atoms and the extra charge. Note lewis structure does not attempt to explain the geometry of molecules, how the bonds form, or how the electrons are shared between the atoms. The calculator will generate the lewis structure for known isomers along with the bonds, ionic charge, formal charge, oxidation numbers and valence electrons for each atom. Be sure to include all resonance structures that satisfy the octet rule. · explain what is a resonance structure and what is not. Draw the lewis structure for the polyatomic trisulfide (s32−) anion. A lewis structure is a way to show how atoms share electrons when they form a molecule. Learn how to find valence electrons and lone pairs. The three s-atoms are arranged linearly in the … Arrange the sulfur atoms in a … What is lewis dot structure of atoms, ions, and molecules. · the lewis structure of the trisulfide anion, s3 (2-), contains three s atoms, with a bent arrangement and bonds between them, suggesting a symmetrical distribution of charge. Explain the octet rule and its limitations, draw lewis structures of simple molecules; Understand kössel-lewis approach to chemical bonding; S 32- represents the trisulfide anion, commonly known as polyatomic trisulfide. This structure is … It consists of three sulfur (s) atoms. Additionally, calculate the formal charge for each atom and discuss the … · the s32- lewis structure is a fundamental concept in chemistry, depicting the arrangement of valence electrons around sulfur atoms in a polyatomic ion. · the calculator will generate the lewis structure for known isomers along with the bonds, ionic charge, formal. Using the lewis structure for s32, explain the molecular geometry and bond angles of this compound. It is the simplest and most limited … · introduction to lewis structures lewis structures are graphical representations of molecules that illustrate the arrangement of atoms and the distribution of electrons within … So32- lewis structure, molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Lewis structures show all of the valence electrons in an atom or molecule. Explain the formation of different types of bonds; Resonance is a way of describing delocalized …
S32 Lewis Structure Explained Beyond The Textbook
Then, learn how to draw the lewis structure. · to draw the lewis structure for the trisulfide anion (s₃²⁻), count a total of 20 valence...