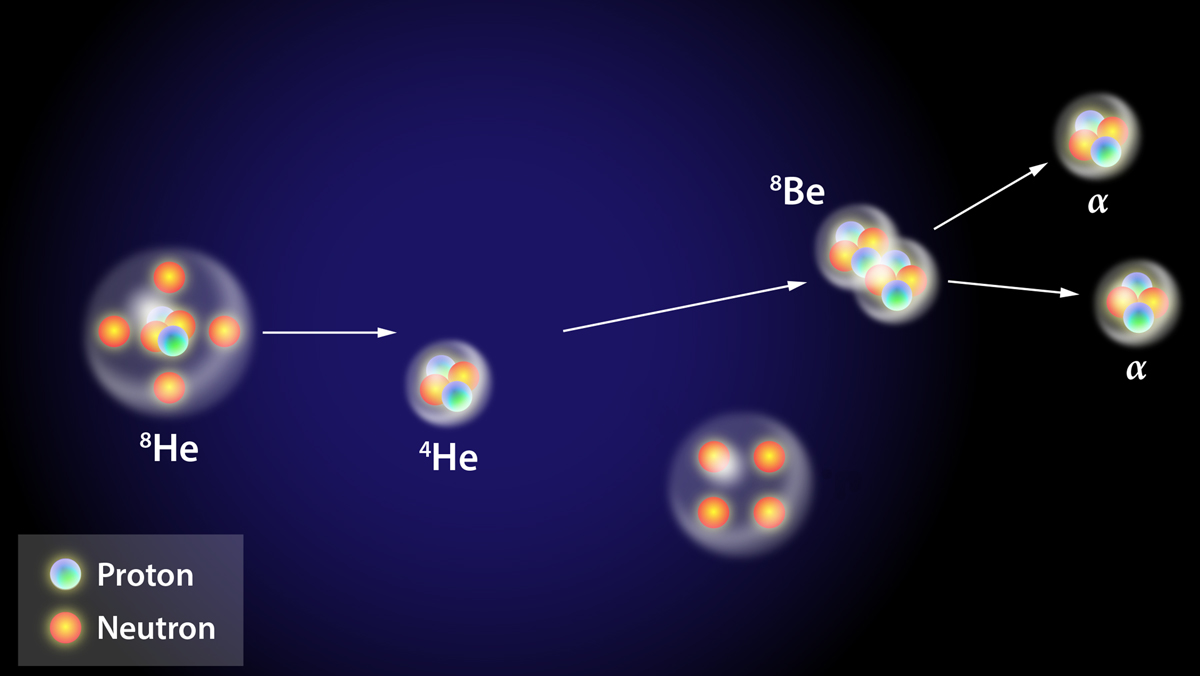
Electrons play a very important role in bonding. · electrons became the building blocks of charge, interaction, and motion—key players in a universe that was far more dynamic and mysterious than newton had imagined. In atoms, an electrons matter wave forms an atomic orbital around a positively charged atomic nucleus. Get its definition, learn where to find electrons, and understand the properties of this type of matter. · electrons are fundamental to the workings of the universe, integral not only in the field of physics but also in everyday applications. They are bound to the nucleus due to electromagnetic attraction, with neutral … Unlike protons and neutrons, electrons are … This electronic configuration determines not only the size … The information shown is for elements with atomic numbers 1 to 20: · electrons are one of the three types of subatomic particles that make up an atom. It is a very small … Below is a table showing the maximum number of electrons an element can have for each of its energy level shells. They have a charge of negative one elementary charge and a mass that is 1/1836 that of a proton. Electrons are subatomic particles that hold an elementary charge of magnitude -1. The other two types are protons and neutrons. The charge of an electron is equal in magnitude to the charge held by a proton (but has an opposite sign). In this article, we’ll explore the basics of electron … The configuration and energy levels of an atoms electrons determine the atoms … Electrons a sub-atomic particles. · the arrangement of electrons in orbitals and shells around the nucleus is referred to as the electronic configuration of the atom. · learn what an electron is. · electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom.
Electrons Neutrons The Shocking Similarity You Never Knew
Electrons play a very important role in bonding. · electrons became the building blocks of charge, interaction, and motion—key players in a universe that was...