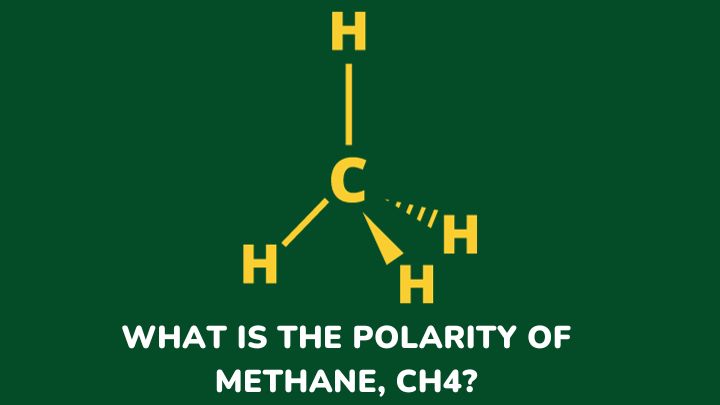
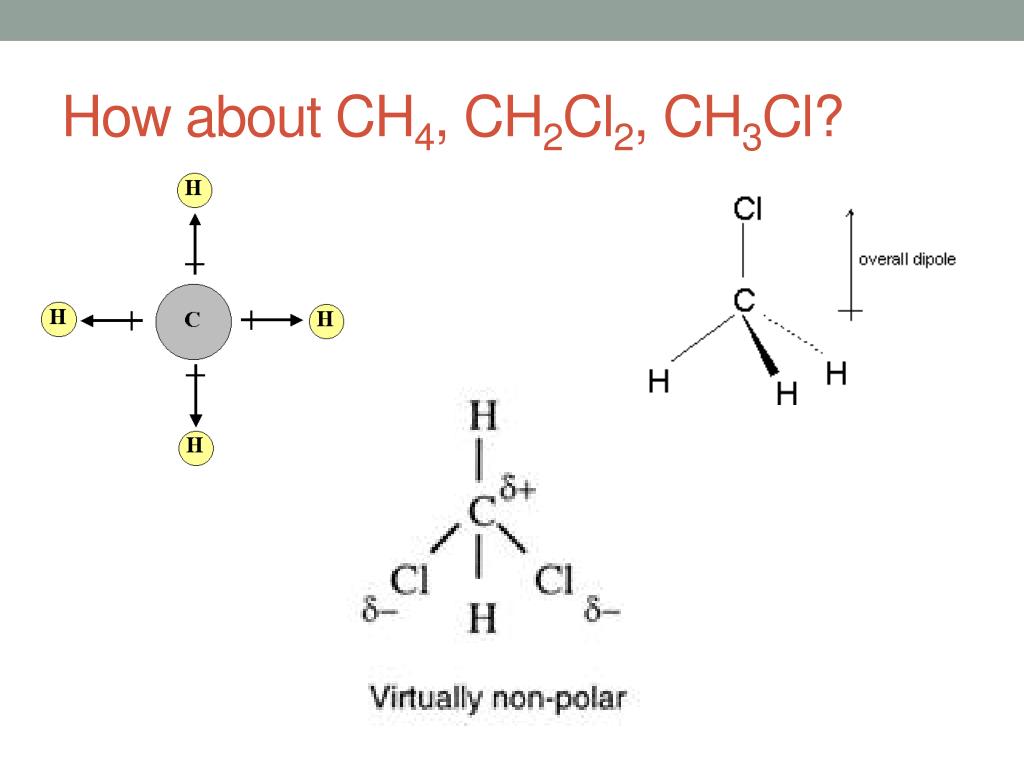
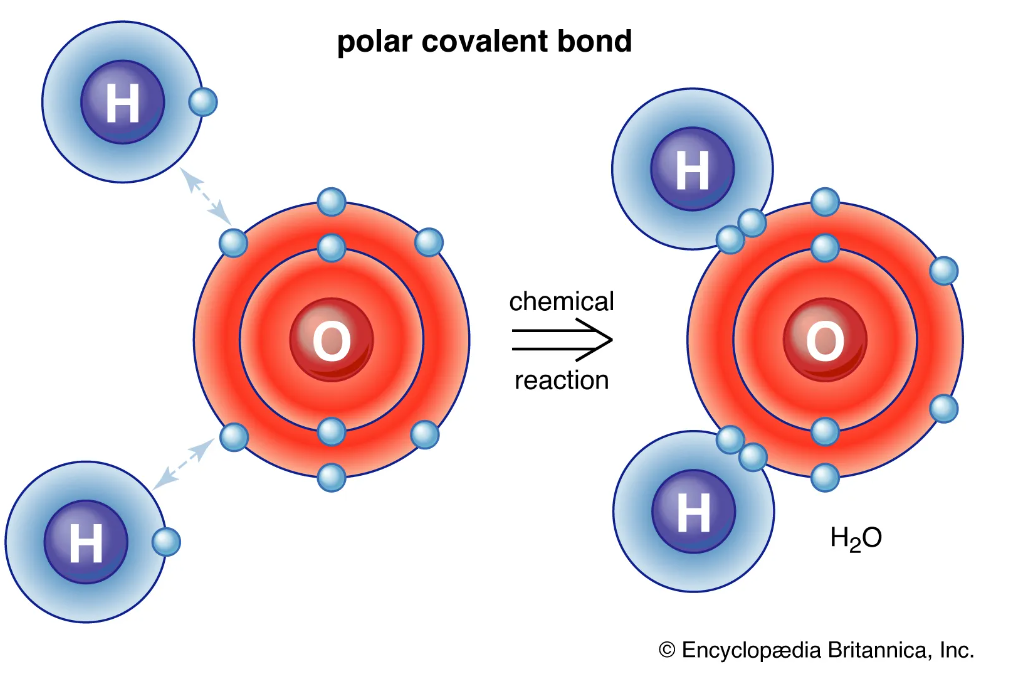
So there are a number of ways to draw the lewis structure for ch4o in which each of the atoms has a full outer shell … Stuck on a stem question? To construct the lewis structure for ch4o (methanol), we will f. · learn about polarity and dipole moments for your a-level chemistry exam. Post your question and get video answers from professional experts: · in ch 4 o, the oxygen that is bonded to the carbon is very electronegative, and the hydrogens that are bonded to the carbon are not very electronegative. For the ch4o lewis structure, we have 14 valence electrons. There are several possible lewis structures for ch4o. In a polar bond, one atom is positively charged and the other is negatively charged. · follow this step-by-step process to find the lewis structure for any molecule. · a step-by-step explanation of how to draw the ch4o lewis dot structure. Follow this step-by-step process to find the lewis structure for any molecule. Find information on molecular polarity, electronegativity, and dipole moments. This creates a more … Since there is an … · to find out the reason why the molecule is nonpolar, let’s go through the factors that help us determine the polarity of the molecules. A step-by-step explanation of how to draw the ch4o lewis dot structure. To determine if a molecule is polar or … Regarding polarity, the difference in electronegativity between carbon and oxygen creates a dipole moment because oxygen is more electronegative than carbon. A molecule (or polyatomic ion) is polar when one side of the molecule is more positive (or more negative) …
Ch₄O Polarity Solved Simple Explanation
So there are a number of ways to draw the lewis structure for ch4o in which each of the atoms has a full outer shell...